More About Standard Additions
Standard Additions is defined in the US EPA ILM040 as being the addition of three increments of a standard solution (spikes) to sample aliquots of the same size. Measurements are made on the original and after each addition. The slope x-intercept and y-intercept are determined by least squares analysis. The analyte concentration is determined by the absolute value of the x-intercept. Ideally the spike volume is low relative to sample volume (approximately 10% of volume). Standard additions may counteract matrix effects but will not counteract spectral effects.
You can select standard additions as the calibration mode on the Standards page of the worksheet.
This page provides information on the following:
- When to use standard additions
- Disadvantages of standard additions
- How does standard additions work
- Blank subtraction
- Tips and tricks
When to Use Standard Additions
- When you cannot suppress physical or chemical interferences in the sample matrix.
- When you don't have a blank and therefore you cannot matrix match sample with standards.
Disadvantages of Standard Additions
- It requires sufficient sample solutions as these need to be subdivided into portions.
- It presumes that you have knowledge of the appropriate analyte concentration.
How Does Standard Additions Work?
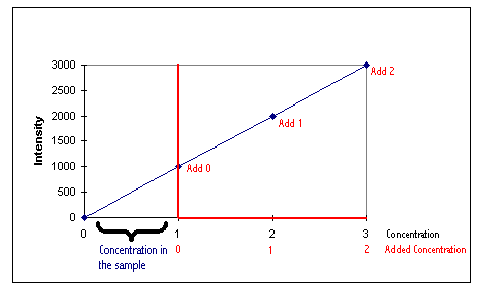
The graph below will be used to illustrate the principle of standard additions, with the quantitative calibration shown in blue.
 |
The instrument response must be a linear function. |
Assume the sample of unknown concentration contains 1 ppm of the element of interest. For Standard Addition purposes the y axis will be positioned at this unknown concentration (1 ppm). This position is labeled Addition 0. A spike of 1 ppm is added to the sample. The total concentration of this sample is 2 ppm and will be labeled Addition 1. An additional spike of 2 ppm will be labeled as Addition 2 and so on. The calibration graph can be interpolated back to the x axis. The magnitude of this result is the unknown concentration of the sample, which is known to be 1 ppm.
Blank Subtraction
If there is a contamination in your reagents the signal of the analyte of interest will be increased and its position on the calibration graph will be changed. This will result in an incorrect interpolated result of the sample concentration. Thus, a blank correction is required.
Tips and Tricks
When using Standard Additions, please note the following:
- Sample 1 equals Addition 0.
See also: