Multimode Sample Introduction System (MSIS)
The MSIS is used with the ICP-OES instrument to simultaneously measure very low trace concentrations of several hydride forming elements. The MSIS mixes both samples and liquid reagents together. The gaseous reactions products are swept away by a flow of nitrogen into the ICP-OES.
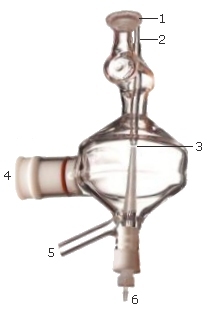
Where:
|
1 |
Ball joint that is secured to the torch with a torch clamp |
|
2 |
Top solution introduction |
|
3 |
1-3 mm gap between the top and bottom solution introduction tubes |
|
4 |
Socket for nebulizer |
|
5 |
Drain |
|
6 |
Bottom solution introduction |
Introduction
The MSIS combines both the nebulization and vapor generation functions and can be operated as either a conventional spray chamber and/or a vapor generator device. Separation of volatilized gaseous hydrides and enrichment of the analyte elements reduces or even removes interference.
Modes of operation
Conventional nebulization
The pump tubing connected to both the top an bottom of the spray chamber are blocked off. Solution and gas are introduced through the nebulizer.
Vapor generation mode
Solution is pumped in from the bottom and reductant is pumped in from the top. The solutions then mix in the 1-3 mm gap between the top and bottom tubes. The pump tubing on the nebulizer is blocked off.
Dual mode
The MSIS is operated in a combined nebulization and vapor generation mode. Sample is pumped into both the nebulizer and bottom port of the spray chamber. Reductant is pumped into the top port of the spray chamber. The solution introduced from the bottom port is mixed with the reductant at the gap between the solution introduction tubes to generate hydrides.
Installation
To install the MSIS:
- Follow the instructions included with the MSIS to connect the fittings and tubing to the MSIS.
- Insert the nebulizer into the socket on the spray chamber.
- The MSIS is mounted onto the ball joint end of the torch with a torch clamp.
- Connect the tubing to a 5-channel peristaltic pump to deliver solutions and pump out the waste from the chamber.
Operation
General procedures
The technique demands a particularly high standard of care in all of the activities which affect the accuracy and precision of the analytical result. Scrupulous cleanliness is essential in all laboratory procedures; standards and samples must be meticulously prepared and carefully handled. Strict precautions must be taken to avoid contamination of apparatus and even if laboratory ware is stored under ideal conditions, it should be thoroughly re-washed before use.
Strict care should also be taken to avoid contamination of all reagents and distilled water. Ideally, reagents should be entirely free of the element of interest, but this is obviously impossible for all analyte elements in all reagents. Consequently, you must always establish the level of the analytical signal attributable to analyte in the reagents. It is standard practice to check the analytical signal from the blank solution before calibrating the instrument and carrying out the analytical program. With the MSIS, however, this procedure must be extended to include the acid and sodium borohydride solutions pumped through the system.
Dispose of waste in accordance with relevant safety practices and local government requirements.
Standard preparation
Prepare your calibration standards from 1000 mg/mL stock solutions.
For some samples, it will be necessary to compare the calibration slopes using the normal calibration method with those obtained using the standard additions method. If the slope is not the same, you should use the standard additions technique for the analysis.
Reductant—sodium borohydride
The recommended sodium borohydride (NaBH4) concentration is 0.6% w/v. However better results will be obtained for difficult samples containing high concentrations of metals if the sodium borohydride concentration is reduced to 0.3% w/v.
 |
Stannous chloride reductant is recommended for mercury determinations. Refer to Mercury. |
Always stabilize the solution by first adding sodium hydroxide (NaOH, 0.5% w/v). Since sodium borohydride will decompose significantly in one or two days, you should not prepare more than 500 mL at a time. At a flow rate of about 1 mL/minute, this should be enough for continuous operation over a typical working day. Stability may be improved by passing the solution through a 5 micron filter. You can also extend the working life of the solution by storing at 5 °C. (The solution should remain stable for approximately one week if stored at 5 °C.) Allow the solution to reach room temperature prior to analysis.
Pump tubing
The pump tubing should be checked regularly by checking the flow-rate (refer to Pump adjustment).
 |
When concentrated acid is first pumped through the acid tube, the inside of the tubing may turn white, but this does not impair the efficiency of the tube. |
Samples, reagents and standards should all be at room temperature prior to analysis. The ICP-OES peristaltic pump will not operate correctly with hot or cold solutions since the pumping rate may vary with solution temperature.
Basic methods
The hydride-forming elements may exist in more than one oxidation state in samples and standards. The following analytical methods for sample and standard have been developed to ensure samples and standards are present in the correct oxidation state.
 |
The practice of including 1% KI in the reductant has been recommended in the past. Adding 1% KI to the samples in a pre-analysis reduction step has since been shown to give better results. Adding the KI to the reductant is no longer recommended. |
Antimony
Prepare samples in at least 1 M hydrochloric acid and ensure that any analyte present as Sb(V) is reduced to Sb(III) by the action of potassium iodide at a concentration of 1% w/v. Reduction is spontaneous and heating should not be required.
| Reductant container: | NaBH4 0.6%, NaOH 0.5% |
| Acid container: | 5–10 M HCl |
Arsenic
 |
See also Additional notes. |
Arsenic in the sample must be in the inorganic form, otherwise digestion will be necessary.
If digestion is necessary, use acid digestion, ensuring no residual oxidizing acid is present, or ashing with an appropriate ashing aid. Simple dry ashing is not recommended.
Prepare samples in at least 1 M hydrochloric acid.
Ensure that any analyte present as As(V) is reduced to As(III) by the action of potassium iodide at a concentration of 1% w/v.
Reduction will take about 50 minutes at room temperature. The reduction can also be carried out at 70°C in about four minutes; however you must cool the samples and standards to room temperature prior to analysis. Since the pumping rate may vary with solution temperature, the ICP-OES pump will not operate correctly with hot solutions.
If the reduction step is omitted, and the analyte is retained as As(V) , the analytical sensitivity is about 20–30% of that obtained for As(III). If the original solution contains As(III) , reduction by potassium iodide is not required.
| Reductant container: | NaBH4 0.6%, NaOH 0.5% |
| Acid container: | 5–10 M HCl |
Bismuth
The presence of potassium iodide will severely suppress the analytical response. Always ensure that the MSIS is completely free of potassium iodide before performing bismuth determinations.
 |
To avoid contamination, it is recommended that you dedicate a separate sample introduction system to the analysis of those elements requiring pre- reduction with KI–As, Sb, Se. |
Prepare samples in 1 M hydrochloric acid.
| Reductant container: | NaBH4 0.6% NaOH 0.5% |
| Acid container: | 5 M HCl (Higher acid concentrations will depress the analytical signal.) |
Mercury
Traces of potassium iodide will interfere severely with the production of mercury vapor and the analytical response may be completely suppressed. Always ensure that the MSIS is completely free of potassium iodide before performing mercury determinations.
 |
To avoid contamination, it is recommended that you dedicate a separate sample introduction system to the analysis of those elements requiring pre-reduction with KI. |
The mercury in the sample must be in the inorganic form, otherwise digestion or use of releasing agents (e.g. CdCl2), will be necessary.
If digestion is necessary, use acid digestion, or ashing with an appropriate ashing aid. Simple dry ashing is not recommended as the mercury will be lost.
Dilute mercury solutions tend to be unstable; all analytical solutions should be stabilized by the addition of nitric acid (5% v/v) and hydrochloric acid (5% v/v). Prepare fresh standards daily.
It is recommended that mercury be determined using stannous chloride as the reductant.
| Reductant container: | SnCl2 (25% w/v) in HCl (20% v/v) |
| Acid container: |
H2O |
 |
To prepare the SnCl2 solution, add concentrated HCl directly to the solid SnCl2 and warm the mixture on a hot plate to complete dissolution before the water is added. |
 |
Mercury suffers from memory effects as it adheres to plastic. Keep concentrations low to minimize these memory effects. |
In this method, it is preferable to use a lower concentration of sodium borohydride than would normally be used for the hydride-forming elements. The concentration of acid pumped through the MSIS is generally not critical.
Ensure the spray chamber drain is clamped to avoid loss of the mercury analyte.
| Reductant container: | NaBH4 0.3% NaOH 0.5% |
| Acid container: | 5 M HCl |
Note that a lower analytical signal may be obtained with this method than with the stannous chloride method.
Selenium
 |
See also Additional notes. |
The selenium in the sample must be in the inorganic form, otherwise digestion will be necessary.
If digestion is necessary, use acid digestion, or ashing with an appropriate ashing aid. Simple dry ashing is not recommended because selenium is highly volatile and recovery will be poor.
Se(VI) is not quantitatively recovered by hydride generation and must be reduced to Se(IV) by warming with concentrated hydrochloric acid. Prepare the samples in 6–7 M hydrochloric acid (use of a lower acid concentration will result in greater inter-element interferences), heat at 70–90°C for at least ten minutes. Cool to room temperature before analysis.
| Reductant container: | NaBH4 0.6% NaOH 0.5% |
| Acid container: | 10 M HCl |
Tellurium
The presence of potassium iodide will severely suppress the analytical response. Always ensure that the MSIS is completely free of potassium iodide before performing tellurium determinations.
 |
To avoid contamination, it is recommended that you dedicate a separate sample introduction system to the analysis of those elements requiring pre-reduction with KI. |
Te(IV) is not quantitatively recovered by hydride generation and must be reduced to Te(IV). Prepare the samples in 6–7 M hydrochloric acid, heat at 70–90°C for at least 10 minutes and cool to room temperature before analysis.
| Reductant container: | NaBH4 0.6%, NaOH 0.5% |
| Acid container: | 5 M HCl |
Tin
The best results for tin will generally be obtained from solutions prepared in 1% tartaric acid. Also, a study has shown the addition of L-cysteine greatly improves the determination of tin by hydride generation.2 The L‑cysteine reduces metal interferences and improves precision and sensitivity. Some improvement in calibration linearity has also been noted.
The concentration of acid pumped into the MSIS is also critical in tin determinations, and the analytical signal will be severely depressed at concentrations higher than 0.5 M.
| Reductant container: | NaBH4 0.6%, NaOH 0.5% |
| Acid container: | 0.5 M HCl |
| Sample: | 1% tartaric acid, controlled pH (2.0–3.0) |
Alternative method
| Reductant container: | NaBH4 0.5%, NaOH 0.1% |
| Acid container: | D.I. H2O |
| Sample: | 1% HNO3, 1% L-cysteine |
Additional notes
- If arsenic and selenium are both to be determined from the same sample, determine selenium first, and avoid KI in samples or standards. You can then determine arsenic after the KI reduction step and any other appropriate treatment (such as the addition of urea if excess nitric acid is present).
- For the determination of arsenic and selenium in many practical samples containing high concentrations of metals such as copper, iron or nickel, fewer interferences have been observed using 0.3% w/v NaBH4 solution concentration (rather than 0.6% w/v). Use of the lower reductant concentration may; however, be less sensitive.
- You can minimize interferences of transition metals such as cobalt, copper, iron, and nickel on arsenic and selenium by preparing samples in 6–7 M HCl. With lower acid concentrations, greater inter-element interferences are present.
- Co-precipitation methods using lanthanum compounds have also been found to be useful.
References
- Smith, A.E., ‘Interferences in the determination of elements that form volatile hydrides with NaBH4 using AAS and Ar/H2 flame.’, Analyst, May, 1975, 100, 300–6.
- AA Application Note, 1992, October, No. 107.
Additional references
AA Application Notes, Numbers, AA: 38, 44, 50, 51, 56, 60, 65, 78, 82, 86, 87, 105, 107 (available for download from the Agilent Technologies website at www.agilent.com)
Spectroscopy, 1985, 1(0), 60
Applied Spectroscopy, 1985, 39(1), 48
Maintaining Tubing
Pump tubes
When the system is not being used, the pump pressure bar should be released, the pump tubes removed from around the rollers and released from the retaining bracket. This will minimize distortion of the tubes and help to prolong their working life.
To reduce mechanical wear of the pump tubes, spray the outside of the tubes and the surface of the pump rollers daily with a silicone lubricant.
The efficiency of all pump tubes will eventually be degraded to the point at which they must be replaced. You should regularly monitor the performance of each pump tube using flow rate measurements (see Pump adjustment). Discard the pump tubes as soon as the results of this monitoring become unacceptable.
 |
The tubes connecting the black/black pump tubes to the peristaltic pump may occasionally need to be replaced. To do this, cut four 2 cm lengths of the thinner black fluoro-elastomer tubing supplied. Place these over each end of two of the black/black pump tubes. |
Fluoro-elastomer tubing
The fluoro-elastomer tubing connecting the gas/liquid separator to the nebulizer is particularly susceptible to contamination with potassium iodide. To avoid this, it is recommended that you dedicate a separate sample introduction system to the analysis of those elements requiring pre‑reduction with KI.
The cleaning procedure for the fluoro-elastomer tubing is as follows:
If there is no KI contamination, you can clean the tubing by flushing it well with distilled water.
If there is KI contamination, you need to remove traces of KI as follows:
- Disconnect and remove the tubing.
- Soak the tubes in sodium hydroxide solution (0.5% w/v). Wash thoroughly with dilute hydrochloric acid, then wash thoroughly with distilled water.
- Allow the tubes to air dry in a dust-free location.
- Reconnect the tubing.
An alternative method of removing traces of iodine from the tubing and gas liquid separator is to pump a freshly prepared 1% sodium thiosulfate solution through the system for 5–10 minutes. You must then remove the thiosulfate by pumping distilled water through the system for 5–10 minutes.