Setup and Run a Method with Expanded QC or Expanded QC with USP 232/233 and ICH Q3D Support
Click File > Options > Expanded QC to configure settings to validate and run methods in compliance with United States Pharmacopeia (USP) <232>/<233> and International Council for Harmonisation (ICH) Q3D requirements.
 |
Administrator rights are required to change the Expanded QC and USP 233/ICH Q3D settings. |
For detailed information on how to set up an Expanded QC run or an Expanded QC with USP <232/233> and ICH Q3D requirements run see the following videos.
Expanded QC
Enable expanded QC functionality on the Options page
Click File > Options > Expanded QC > Enable expanded QC functionality to add samples and default All Sample QC (ASQC) levels to the QC table. This will be used to compare samples against expected analyte levels.
Creating sample types on the Options page
For a demonstration, click 'Enable and Configure Expanded QC' in the 'Setup and Run a Method with Expanded QC' video.
- Enter the sample type name and then click the + button.
- Repeat for all sample types.
- Enter the QC limits based on an expected range for the elements within the sample.
- Click OK.
Selecting the QC tests on the QC page
For a demonstration, click 'Setup QC Tests' in the 'Setup and Run a Method with Expanded QC' video.
All Sample QC definitions (ASQCs) will be assessed for all samples of a specific, user-definable sample type in a worksheet.
If the Sample type is left as 'All-Samples' (defined on the Sequence page), then all samples in the Sequence table will be assessed on this QC definition, regardless of the Sample Type selected.
If the Sample type is changed (Figure 1), then the Sample type must also be changed on the Sequence page (Figure 2) to apply this QC definition to that sample.
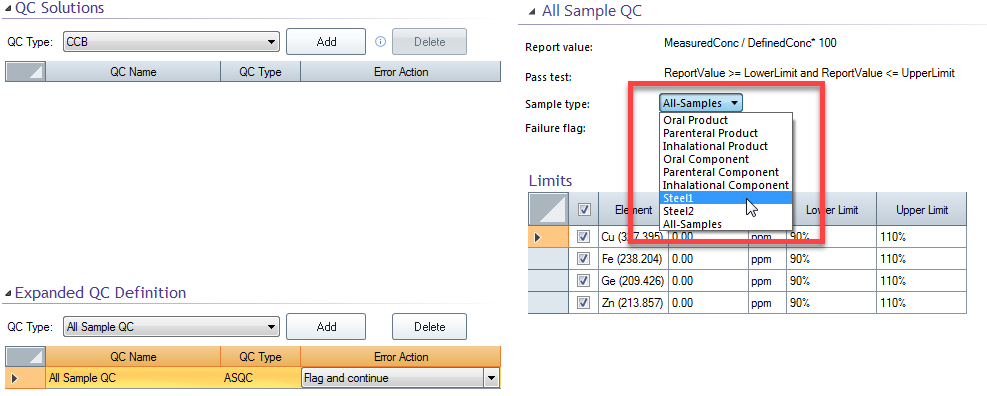
Figure 1. Selecting Steel1 on the QC page where the Steel1 sample was created on the File > Options > Expanded QC page.
Selecting the Sample type on the Sequence page
For a demonstration of how to add QC types and change the Sample type, click 'Setup the Sequence' in the 'Setup and Run a Method with Expanded QC' video.
- Customize your view and report by adding or removing additional sample data columns.
- Select the sample type to associate with the specific QC definition created on the QC page.
QC definitions that have the sample type 'All-samples' are applied to all samples in the sequence regardless of which sample type is selected on the Sequence page.
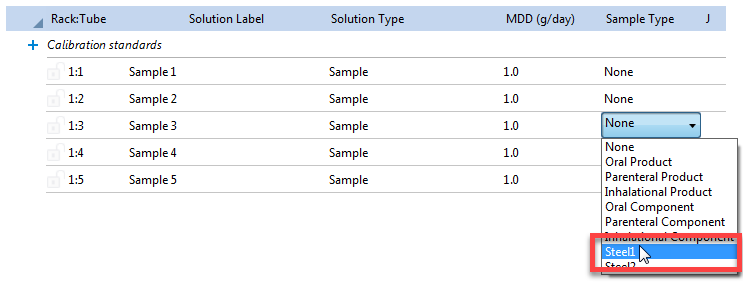
Figure 2. Selecting the Sample type on the Sequence page.
Setup a Method with Expanded QC with USP and ICH Requirements
 |
Administrator rights are required to change the Expanded QC and USP 232/233 and ICH Q3D settings. |
Enable USP 232/233 and ICH Q3D-specific support on the Options page
Click File > Options > Expanded QC > Enable USP 232/233 and ICH Q3D-specific support to populate the table with USP232 and ICH Permissible Daily Exposure (PDE) levels and enable USP232 and ICH-specific QC within worksheets.
 |
For detailed information on definitions and requirements, go to the USP (http://www.usp.org) and ICH (www.ich.org) websites. |
All tests are pre-populated with the recommended values from the USP 232/233 and ICH Q3D Method Validation Test limits. The limits are editable.
Adding sample types or changing PDE values
- Enter the name and then click the Add (plus sign) icon to add a sample type or change existing PDE values if 'Enable USP 233/ICH Q3D specific support' is selected.
- Enter or edit the permissible daily exposure limits.
To remove a sample type, select the appropriate column in the table and then click the Remove (minus sign) icon.
Using expanded QC lets you select from the standard QC definitions to help you validate and run methods in compliance with the USP <232/233> and ICH Q3D requirements and assign them to specific sample types.
A pass/fail is assigned based on the ‘J’ value.
The 'J' value is calculated based on the specific drug MDD (maximum daily dose), as well as the sample preparation parameters (weight, volume and dilution) in combination with the PDE for each element being analyzed.
Selecting the QC tests on the QC page
When adding USP 232/233 and ICH Q3D support, the equations are not editable and are defined by the USP <232/233> and ICH Q3D requirements.
With the exception of 'LSpike', the only error flag available is 'Flag and continue'.
All definition limits are pre-populated with recommended USP and ICH requirements, however, they are editable.
 |
For detailed information on definitions and requirements, go to the USP (http://www.usp.org) and ICH (www.ich.org) websites. |
About USP 232/233 and ICH Q3D QC types
Component
The summation option measures impurities in drug components (raw materials, excipients and APIs) and calculates the elemental impurities in drug products based on amount of impurities in drug components and percentage of each component in the drug product.
Control Threshold
Select to check that the elemental impurity level is less than the control threshold (defined as being 30 percent of the established PDE in the drug product). If the check indicates that the elemental impurity level is more than the control threshold, additional controls should be established to ensure that the elemental impurity level does not exceed the PDE in the drug product.
LSpike
For an example of setting up LSpike and using the Spike Calculator, click 'Setup QC Tests' in the 'Expanded QC with USP 232/233 and ICH Q3D Requirements' video.
LSpike is the spike percentage of the J value*. Typical use is for 50%, 100% and 150% J spikes; however, you can select up to 8 spike levels. The number of spikes (the Spike level), is set in the File > Options > Expanded QC page.
The Spike Calculator  (next to the Limits table) determines the concentration needed to use for spikes of varying percentages of J. The actual concentration that is '1.00J' in a given solution depends on the weight, volume and dilution from sample preparation, and the max daily dose and administration route for the specific drug.
(next to the Limits table) determines the concentration needed to use for spikes of varying percentages of J. The actual concentration that is '1.00J' in a given solution depends on the weight, volume and dilution from sample preparation, and the max daily dose and administration route for the specific drug.
*The J value is defined as the PDE concentration of the element of interest at the target limit, appropriately diluted to the working range of the instrument.
Product
The drug product option measures the amounts of each element in the drug product and compares results scaled to maximum daily dose with the modified daily dose PDE. The measured amount of each elemental impurity scaled to the daily dose must not be more than the specified PDE.
Limits
The recommended limits set by the USP and ICH are pre-populated but are editable.
Selecting the Sample type on the Sequence page
For a demonstration of how to add QC types and change the Sample type, click 'Setup the Sequence' in the 'Expanded QC with USP 232/233 and ICH Q3D Requirements' video.
- Customize your view and report by adding or removing additional sample data columns.
- Select the sample type to associate with the specific QC definition created on the QC page.
QC definitions that have the sample type 'All-samples' are applied to all samples in the sequence regardless of which sample type is selected on the Sequence page.

Figure 2. Selecting the Sample type on the Sequence page.
For detailed information on how to set up an Expanded QC run or an Expanded QC with USP <232/233> and ICH Q3D requirements see the following videos.
See also: